Why Do Small Engines Suffer From Ethanol Problems? +VIDEO and SPECIAL UPDATE
 |
HINT - They don't!
By Marc J. Rauch
Exec. Vice President/Co-Publisher
THE AUTO CHANNEL
Originally published September 1, 2015
Updated September 29, 2019
 Marc Rauch with Aircraft Carrier MIDWAY in San Diego |
I recently responded to a knucklehead online radio broadcast that featured Lauren Fix bashing ethanol, something she has done in the past even though ethanol is something that she seems to be completely unable to discuss with any accurate information. The broadcast was an episode of "The Jacki Daily Show," hosted by Jacki Pick. The complete episode and my comments can be found by CLICKING HERE.
But this editorial is not really related to Ms. Pick's interview of Ms. Fix, it concerns a short comment made at the beginning of the show as Jacki Pick read an email that was supposedly written by a male colleague of hers. This is what she read on-air:
- "I ruined a weed trimmer and a chainsaw on
this stupid E15 ethanol gasoline."
The alleged writer of the email didn't offer any further details of what he meant by "ruined," and Ms. Pick didn't elaborate any further on the issue other than to use it as the springboard to introduce Lauren Fix to the show.
However, this comment is just one instance of negative claims made against ethanol (alcohol fuel) in regard to small internal combustion engines (two-stroke engines). These are the engines typically found in lawn and garden equipment, motorcycles, and boats.
There are three complaints made about ethanol-gasoline fuel blends. The first is that the ethanol causes sludge, tar, gunk, goo, crap (or whatever you want to call it) to appear in the fuel tank, thereby clogging the filter, spark plugs, fuel injectors, carburetors, etc.
The second complaint is that the ethanol corrodes the rubber hoses and some metal parts.
The third is that the ethanol in the fuel-blend magically sucks water right out of the air and drops it in your fuel tank.
Of the three, I'd say that the first - "the sludge quagmire" is the most often heard. And without knowing anything further about what Jacki Pick's colleague was referring to, I'd say that this is what he might have meant - assuming that the email was real and not just a device to open the show.
To get to the bottom of this issue, basic questions must be asked:
Question #1 - Does sludge/goo/gunk/crap form in an internal combustion engine? The answer is yes.
Question #2 - Is the sludge/goo/gunk/crap because of ethanol? The answer is no; it is because of the gasoline and oil used in the fuel.
While ethanol is a fuel, it is also a very effective solvent. Solvents are used to clean and remove the residue and deposits left after a substance has been used, such as the residue and deposits left after gasoline and/or oil is burned.
Gasoline is also a solvent, but it's not as effective a solvent as ethanol. Gasoline and petroleum oil products are, however, very effective in leaving residue. They are champs in leaving behind deposits.
In short, if you were choosing up sides to compete in a national residue-making competition, you would be better to select gasoline or petroleum oil for your team. But if you were choosing teammates for a national clean-up competition, you'd be better off to pick ethanol.
How much more residue does gasoline or a gasoline-oil combination create as compared to ethanol? Watch this video from 2009 to find out:
Now, imagine that the glass jars shown in the video are the insides of an internal combustion engine. Imagine, if instead of burning just fuel-soaked wicks for a few seconds, you were to burn the fuel-soaked wicks for an hour or two every day for a week, a month, a year. Based on the results of the video, could you imagine that the residue and deposits from the gasoline and gasoline-oil fuels would be caked on? Would the residue be a half-inch deep? Would it be 1 inch deep? More than 1 inch?
Would the caked on residue affect the functionality of an engine's pistons? Would the solid caked-on bits break off and affect the functionality of other parts of the engine? Of course it would. This is one of the reasons why engines fail and why mechanics have to clean out an engine. To mitigate this problem gasoline companies add "detergents" to the gasoline to help dissolve the build up. If gasoline was a more effective solvent it might be able to remove the residue that used-gasoline creates.
In an article published by GasBuddy.com on June 29, 2013; Gregg Laskoski wrote about automakers' efforts to
encourage the development of more effective gasoline detergents. Mr.
Laskoski is Sr. Petroleum Analyst at GasBuddy and, interestingly enough,
had been Managing Director of Public Relations at AAA Auto Club South.
Here's are some excerpts from that article:
- "The automakers formed a consortium, Top Tier Gasoline, that certifies
retailers as meeting their standard for detergent. They say it's necessary
because detergents prevent deposits of leftover material from building up
in engines and exhaust systems. The deposits are like the ash that remains
in a fireplace, and their presence in your car's engine can reduce fuel
economy and performance."
"We strongly recommend Top Tier detergent gasoline to keep your engine clean,” GM fuel specialist Bill Studzinski said. “Fuel economy, emissions and acceleration all suffer when there are deposits in an engine."
I hope you noticed that there isn't one single mention of ethanol in these two paragraphs that attribute the deposits and residue problem to ethanol. And if you have the opportunity to read the entire article you'll see that the word ethanol never appears anywhere in the text of the story.
SWITCHING GEARS
Let me take you in a different direction. Imagine having a fuel that creates much less residue and is a very effective solvent at the same time. In other words, something that might make a small mess, and then cleans up after itself...along with cleaning up the mess left by the petroleum-based fuels. Isn't that better? How could it not be better?
But wait, some engines do have problems with sludge, tar, gunk, goo, crap (or whatever you want to call it) after the fuel has been switched to ethanol or an ethanol blend. There, I said it; I admitted it.
If you could fault ethanol fuels for anything, it's that they are too efficient at cleaning the residue and deposits. But that's like complaining that your toothpaste whitens too much; that your hand soap gets too much dirt off your hands; that your greasy pots and pans clean up too quickly.
 Gasoline damages engines |
If you have an engine that has used some type of gasoline fuel or gasoline-oil fuel then the engine will have substantial deposits throughout, and you will have to have it serviced. You will have to change filters, spark plugs, anything, and everything that comes in contact with the burned fuel deposits. Through the life of the engine and the device that the engine powers, you will have to do this, maybe multiple times.
The good news is that once you have had this service completed ethanol-gasoline fuels will keep your engine clean longer and better than any non-ethanol gasoline with detergent. Mercury Marine, the world's largest manufacturer of boat engines agrees with this. You can watch and listen to their entire Ethanol Seminar webcast by CLICKING HERE.
So if petroleum oil products, like gasoline and diesel, are the cause of the build up of all the engine "ash," or engine "plaque," then why do some people and organizations claim that ethanol is the cause of the problem?
Well, you could say it's a lie to blame ethanol. In fact, I will say it: It's a lie.
You saw the video demonstration above, you read the quotes from the GasBuddy.com article, and if you have just the most rudimentary knowledge of internal combustion engines then you know that what I've written is true. Then why don't people who are supposed to be experts in automotive care not know this very rudimentary information? Shouldn't they be embarrassed to show their faces in public?
...Special Update Coming Up...
DOES ETHANOL DAMAGE RUBBER PARTS AND METAL COMPONENTS?
Automobile and engine parts break. They wear down. They corrode. It's what they do; it's what they've done since movable devices were invented. Human parts do the same, by the way. The auto parts and engine repair industries didn't start with Congress' passing of the Renewable Fuel Standard. There were parts stores all over America and all over the world for dozens and dozens and dozens of years. There have been engine repairman forever. The question is, "Is ethanol more corrosive to engine parts more than gasoline?" The answer is that at the worst, it is no more corrosive than gasoline, and in some instances, it is less corrosive.
If you're prone to dislike ethanol you will now say, "Yeah, then why does my engine mechanic (or father or uncle or husband) say it is?" The easiest answer is that they either don't know, and they're just passing along bad information that they've heard, or that they needed an excuse so they used ethanol as the villain. In short, they misdiagnosed the cause.
At this point, the disbelievers say, "Bu*l Sh*t!"
There's two ways to prove the point. One way is by using a visual presentation of the comparative corrosive effects, and the other is by a scientific comparison. Let's first look at the video tests conducted by Jacob Kaul; you'll see how after just a short period of time fuel line hoses react to being exposed to different fuels:
Here's two videos of long term tests conducted by Erron Spalsbury that prove that ethanol fuels do not cause damage to metals or rubber hoses:
Here's a video about testing E85 in lawn mower engines that was conducted by Owensboro Technical College:
Now let's look at scientific comparisons. A company called Custom Advanced Connections has a website that compares a wide variety of chemicals with different types of rubber (https://www.customadvanced.com).
They provide the results via both a dynamic database and a static list. Some of the direct links to information are:
-
Ethyl Alcohol and Rubber
Denatured Alcohol and Rubber
Gasoline and Rubber
Benzene and Rubber
Toluene and Rubber
Xylene and Rubber
The results show that ethanol is the least harmful compared to gasoline and the various "aromatic" substances. The one type of rubber that shows ethanol to be slightly less compatible, and at the same time shows gasoline and the aromatics to be better than ethanol, is Viton, Fluoroelastomer (FKM). In any event, the rating for ethanol is still in the highly acceptable category, and no where close to the "severe damage - not recommended categories that gasoline and aromatics fall into with natural rubber, silicone, and neoprene, to name a few.
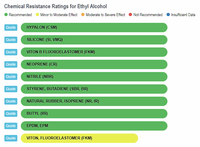 Ethanol vs Rubbers |
 Denatured Alcohol vs Rubbers |
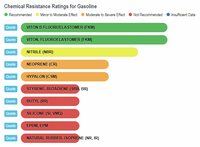 Gasoline vs Rubbers |
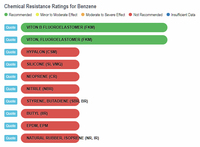 Benzene vs Rubbers |
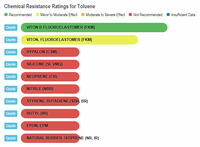 Toluene vs Rubbers |
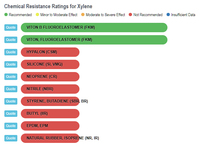 Xylene vs Rubbers |
Viton, Fluoroelastomer is a rubber invented by Dupont in the 1950's and first used in automobiles in the late 1950's. There are two important points about this:
• The first is that Dupont was one of the three partners in the leaded-gasoline patents (with General Motors and Standard Oil), and because of gasoline's - especially leaded gasoline's - corrosive characteristics, Dupont obviously had it in their best interest to develop a rubber that was very resistant to leaded gasoline.
• The second point is that the use of Viton in automobile engine components beginning only in the late 1950's would explain why ethanol and ethanol-gasoline blends in all the previous years never caused any problems, and why there is an absence of reports citing ethanol-related damage during the many decades that ethanol-gasoline blends were extensively used outside of the United States (this is the salient point behind the editorial I published in December 2017 - The Hypocrisy of Big Oil).
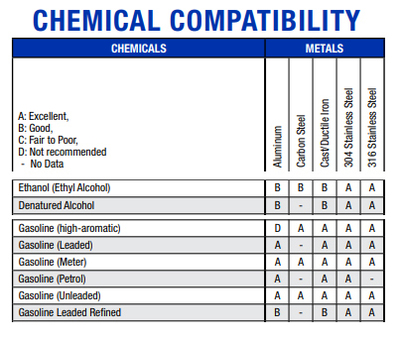
Another website of another company, Graco, Inc., provides a similar comparison, but they also include metal substances. Ethanol is ranked slightly lower than some varieties of gasoline with metals - but still in the
"good to excellent range" - and about equal to benzene, toluene, and xylene. These results and comparisons puts to rest any claim that ethanol is harmful (or more harmful) to metals that might be used in an internal
combustion engine and fuel system.
(http://www.graco.com/content/dam/graco/ipd/literature/misc/chemical-compatibility-guide/Graco_ChemCompGuideEN-B.pdf)
 |
A third resource is the website for CPLabSafety. They specifically analyze and compare different chemicals to aluminum, and it shows similar results to the Graco website metals comparison.
One interesting result is that "high aromatic gasoline" ranks as not recommended for use with aluminum in both the Graco and CalPacLab comparisons.
(https://www.calpaclab.com/aluminum-chemical-compatibility-chart/)
Sometimes, it's claimed that ethanol damages plastics. However, you can store ethanol in ordinary plastic water bottles. Aromatics (benzene, toluene, and Xylene), the substances used to replace ethanol in E0, are far less compatible with different types of plastics. See a comparison chart at http://www.plasticsintl.com/plastics_chemical_resistence_chart.html. The CPLabSafety website confirms ethanol's compatibility with plastics with the charts at https://www.calpaclab.com/chemical-compatibility-charts/.
DOES ETHANOL SUCK WATER OUT OF THE AIR?
No, absolutely not. It is a ridiculous myth. I wrote and published an extensive paper on this issue, which you can find at Ethanol Does NOT Suck Water Out Of The Air.
SPECIAL UPDATE - September 29, 2019
The other day I was reminded of another category of small internal combustion engines that have been used for years by hundreds of thousands, perhaps millions of people around the world on a regular basis. These small 2-stroke and 4-stroke internal combustion engines prove once again that alcohol fuels are safe to use, and that the corrosion problems and debris problems blamed on alcohol fuels are either non-existent or overly exaggerated. These engines are the IC engines used in model cars, airplanes and boats. The engines are miniature versions of full-size engines, and they work in exactly the same manner as their larger counterparts. The fuel they use consists primarily of alcohol (in this case, methanol). The volume of methanol typically runs from about 60% to 95%. This would be equivalent to M60 to M95 (think E60 to E95). The RC fuel usually also contains some nitro - nitromethane - for starting and a small amount of oil for lubrication. The need for the oil is like the use of 2-stroke oil in other small engines such as those in lawn equipment, go-karts, and some ATVs.
RC internal combustion engines, which are simply miniaturized versions of full size engines, are NOT susceptible to methanol corrosion. There is a difference between methanol and ethanol...ethanol is compatible with more materials than methanol...ethanol is even less corrosive than methanol.
RC internal combustion engines do experience problems, but the problems are exactly the same as the problems caused by petroleum oil and gasoline. If the engines are not regularly cleaned (many people say after each use) the oil gets gummy and it can harden into a varnish. The easiest and least expensive solvent to use to clean engines and parts is to use some type of alcohol. So, once again, this proves that ethanol used in ethanol-gasoline blends is not the cause of full size engine problems, it is the solution to many internal combustion engine problems. The typical cause of the problems blamed on ethanol are actually gasoline and aromatics.
Oops, there one other thing to consider. Alcohol can be used to clean sensitive, expensive electronics, too. You can use isopropyl alcohol or ethyl alcohol. Among the items the rubbing alcohol can be used on are video and
audio heads on cameras and tape machines. Typically you would use a swab or a q-tip dipped in alcohol and then rub the alcohol over the item requiring cleaning. The alcohol dries quickly and cleanly, and leaves no sticky
residue. Let me repeat that, the alcohol dries quickly and cleanly, and leaves no sticky residue.
FINAL ANALYSIS
How do the slimebags that work for API get away with this? How do media whores hired by the oil industry get away with repeating information that are ridiculous downright lies? Why are there any small engine mechanics that don't understand this (they should all be at the forefront to argue for ethanol, not against ethanol)? How can you trust a mechanic that can't or won't diagnose a simple problem correctly?
In the broadest terms possible it could be said that all fuels used in any type of engine will contribute to the engine breaking down. It could be a spark-induced internal combustion engine, a compression-ignited internal combustion engine, a steam engine, a water or wind turbine, or an electric motor; they all break down, and the fuel that supplies the power helps to cause it. Engines have moving parts; moving parts wear down. Internal combustion engines suffer from thousands of small explosions per minute.
When the anti-ethanol people talk about a fuel damaging an engine they make it seem as if only ethanol causes the wear and tear. They make it seem as if the gasoline somehow cushions the explosions or coats the engine components to prevent deposits from forming. This is a lie. This lie is told to keep us subservient to the oil industry and the terrorist countries that are the key suppliers. There is no fuel used in an internal combustion engine that is worse for the engine than gasoline. Gasoline damages engines. Small engines suffer from gasoline problems. Ethanol helps to clean the engines.
If gasoline was not harmful to engines, America would not have had thousands of engine repair shops and mechanics spread throughout the country in all the years during the age of leaded gasoline.
Small engines and big engines don't suffer from ethanol problems, they suffer from gasoline problems.
SEE ALSO: HELP! Vandals Are Spraying Ethanol All Over The Neighborhood
Special thanks to Dana Fletcher for his contributions to the information contained in the materials comparison section.


