Ethanol Does NOT Suck Water Out Of The Air +VIDEO
 |
• UPDATED January 24, 2020 with a new bombshell hygroscopic video test that destroys anti-ethanol myths
By Marc J. Rauch
Exec. Vice President/Co-Publisher
THE AUTO CHANNEL
 Marc Rauch |
Well, it's a lie: ethanol does not suck or absorb water right out of the air. The only thing that sucks is that the oil industry has been allowed to propagate lies like this for so long.
The origin of the claim is that ethanol is classified as a "hygroscopic" substance. Please note the spelling: hygroscopic (we've had people send us emails that say "ethanol is hydroscopic..."). It is HYGRO, with the letter "G." It seems that many years ago some clever oil industry person must have learned that ethanol (alcohol) is a hygroscopic substance, and that the general dictionary definition for a hygroscopic substance is that it can attract moisture from its environment.
What the oil industry wag then did was to play a semantic word game by substituting the word "attract" with "absorb," and "air" for
"environment." The words attract and absorb are two different things, with two different meanings:
-
Attract - to pull to or draw toward oneself or itself (as a magnet attracts iron).
Absorb - to take in and make part of an existent whole (as a sponge absorbs water).
And the words environment and air have different contexts:
-
Environment - the immediate adjacent surroundings (as in the type of environment in which you live).
Air - the mixture of invisible odorless tasteless gases that surrounds the Earth.
How do we know that a semantic word game was played and that alcohol will not absorb water right out of the thin or ambient air, simple: fill any open container halfway with alcohol and place it on your kitchen counter. Allow it to sit for one or more days. If alcohol absorbs water right out of the air, then when you check the level of liquid in the ensuing days you would find that the volume of liquid has increased. Try it and see what happens. You can watch my demonstration of this experiment in the video window below.
Incidentally, cotton is also a hygroscopic substance. So just as additional proof that being a hygroscopic substance doesn't mean that it absorbs water right out of the air, place a ball of cotton on the other side of your kitchen counter and see if it gets saturated with water from just sitting out in the open. SPOILER ALERT, the cotton ball will not get saturated with moisture. However, if you want to see a hygroscopic substance in action, then place the cotton ball immediately adjacent to some water, so that the cotton ball is in an environment that includes liquid water. The cotton ball "wicks" up the water. If you place a piece of glass next to the water, instead of the cotton ball, the glass does not wick up the water because glass is not a hygroscopic substance.
Other than by a dumb accident, water will form in your fuel system because of condensation, but condensation has nothing to do with hygroscopic action. For example, if you have a glass-top outdoor table you have probably noticed that moisture formed on the glass after a cool night. This happens to your car windows. Since glass is not a hygroscopic substance, how does this occur? The moisture forms because of condensation.
So what do you do if you have some water in your fuel system? Do you stick a straw in and suck it out? No, you add a product like Dry Gas. Dry Gas is one or more type of alcohol, and ethanol is alcohol, meaning that you use ethanol to solve the problem of water in your gasoline tank. That's right, to solve the problem! In the years since E10 has been in regular use the overall incidence of car owners needing to use Dry Gas has declined dramatically. In April 2003, NPR Radio's Tom and Ray Magliozzi (CAR TALK Click & Clack) responded to a question on this issue:
"Dear Tom and Ray: Every time I fill my gas tank, I buy half a quart of dry gas. My
father says it really helps with the fuel line and keeps things
clean. Is this stuff legit, or am I wasting my cash? -- Chris
RAY: Well, we think you're wasting your cash, Chris. This stuff is
mostly alcohol, which absorbs any moisture that is in your gas tank.
TOM: The idea is that by getting rid of any water in the tank, you
eliminate the chance of the water freezing in the fuel line and
preventing the car from running.
RAY: But to be honest, we haven't seen a frozen fuel line in our
garage in a decade or more. Why? It could be because gasolines are
better-formulated now. All gasolines have alcohol already mixed into
them."
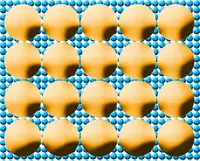 Yellow circles represent ethanol molecules. Blue circles represent water molecules. |
Water is absorbed into ethanol because water molecules are small enough to fit between the larger ethanol molecules. The water molecules are then vaporized in the combustion process. Gasoline molecules, on the other hand, doesn't have the ability to pack as many water molecules around them, so the water separates from the gasoline and freezes, if it's cold enough, or gets sucked into the piston chambers where it cannot easily combust. In other words, ethanol aids combustion, not inhibits combustion.
Does this mean that you could pour five gallons of water into your gasoline tank and the available ethanol will break down all this water? No, don't be silly; too much of anything can be bad...even ice cream.
Want to know what one of America's leading auto mechanics says about ethanol? Read this article from Bobby Likis: Can’t We Just Get Rid of Ethanol Ignorance?
And for those among you who are boat owners and have been swayed by the ethanol-hygroscopic lies related to boats, you should be aware of the Mercury Marine Ethanol Webinar conducted in August 2011 - you will be shocked at their study findings, a position they've continued to support through two subsequent updated Power Point presentations (one in 2016 and another in 2018). The 2016 presentation can be found by CLICKING HERE. And you can watch the 2011 webinar in the video below.
For those of you who are too impatient to see the entire presentations, this is Mercury Marine's finding:
"There is no active transfer mechanism for ethanol molecules to reach out and grab water molecules out of the air. Under normal storage conditions, even in a vented fuel tank it just does not happen...The primary cause of water collecting in fuel tanks is condensation from humid air..."
From this point forward, let there be no more discussion about ethanol sucking the water right out of the air. Those who do, suck.
What About Dessicants?
Almost everyone is familiar with the little silica packettes that are placed in packages to keep the items in the package dry from condensation. These silica packettes are "dessicants." The process by which
dessicants work is called "deliquescence." Dessicants do absorb moisture (water vapor) out of the air. This process has undoubtedly helped to contribute to the misunderstanding of hygroscopy because dessicants
are hygroscopic. However, while all dessicants are hygroscopic, not all hygroscopic substances are dessicants. To understand this better, consider mandarins and tangerines. Mandarins are a type of orange citrus fruit.
Tangerines are also a type of orange citrus fruit, however, they are not the same. All tangerines are mandarins, but not all mandarins are tangerines. Tangerines fall within the overall category of mandarins.
Ethanol and other alcohols are not dessicants, they do not absorb water vapor from the air, they will absorb small amounts of water that they come in direct contact with (per the molecule comparison diagram
shown above).
SEE ALSO:
• The Irrelevance Of BTU Rating - Big Oil's Gimmick To Hoodwink The Public• The Rise & Fall of General Motors and the Subjugation of the Industrialized World
• TRUTH ABOUT ETHANOL - Review and Reply to Robert Bryce's GUSHER OF LIES
 |
For a lot more information about ethanol fuel you can read my 641-page book, THE ETHANOL PAPERS. It's available to read online for free by CLICKING HERE.
ABOUT THE AUTHOR
Marc J. Rauch is a multi-award winning TV/film writer, producer and director and has been a broadcasting and marketing executive for more than 50 years. In 1995, Marc wrote and published "MARCETING - The Book On Effective Advertising and Promotion." This book was updated and re-published in 2005 with additional chapters covering the Internet and online marketing.
In 1987, Marc co-founded The Auto Channel television network, and TheAutoChannel.com a few years later. Marc serves as the company's Executive Vice President and Co-Publisher. TheAutoChannel.com is the Internet's largest and oldest automotive information resource, with more than 1.5 million pages of content. Marc is a leading advocate of alternative fuels and energy; writing, lecturing and making numerous broadcast appearances regarding these issues. He's traveled to Taiwan to speak at the Asia-Pacific Economic Cooperation Forum on behalf of the U.S. Department of Agriculture, he's presented testimony on the use of ethanol before Congressional debriefings in Washington on two occasions, was a keynote speaker at the 2016 Australian National Biofuels Conference, and was a featured speaker at the 2018 National Ethanol Conference in San Antonio, Texas. In 2019, Marc wrote and published "THE ETHANOL PAPERS," a 600-page book that presents the whole story on ethanol fuels. In 2021, Marc published his extended essay "YES, TIN LIZZIE WAS AN ALCOHOLIC" about Henry Ford's Model T in book form and is available from AMAZON BOOKS.

